How to identify if the FDA "certificate" is genuine?
- Categories:Industry News
- Author:woozon Healthcare
- Origin:
- Time of issue:2023-03-03
- Views:0
(Summary description)How to identify if the FDA "certificate" is genuine?
How to identify if the FDA "certificate" is genuine?
(Summary description)How to identify if the FDA "certificate" is genuine?
- Categories:Industry News
- Author:woozon Healthcare
- Origin:
- Time of issue:2023-03-03
- Views:0
United States Food and Drug Administration(FDA),FDA is one of the U.S. government's implementing agencies within the Department of Health and Human Services (DHHS) and the Department of Public Health (PHS).As a scientific regulatory agency, the FDA is responsible for ensuring the safety of foods, cosmetics, drugs, biologics, medical devices and radiological products produced or imported into the United States. Internationally, FDA is recognized as one of the largest food and drug regulatory agencies in the world.
However, what we call FDA certification is not really certification, but rather a registration.
The FDA classifies medical devices into three classes. Class I, Class II, Class III. Class III belongs to the highest subcategory.
Class I products (47%)
General Control The vast majority of products only need to be registered, listed and GMP (good manufacturing practice) to enter the US market.
Class II products (46%)
General control + special control, after registration and listing of these products, in addition to the above general control, most of the other products are required to go through the Premarket Notification (PMN: Premarket Notification) (i.e., 510K). A small number of Class II products may be exempted from the premarket notification process. Manufacturers must file a 510 (K) application with the FDA 90 days before a product can be marketed.
Class III products (7%)
After registration and listing, the company shall implement GMP and submit an application for PMA (Product Marketing Approval Standard) to FDA (part of Class Ⅲ products or PMN). Generally speaking, Class III products are mostly maintenance, life support or implants,hose that may cause injury or disease, such as cardiac rhythm regulators, intrauterine devices and baby incubators, account for about 7% of all devices. These devices must obtain a PMA from the FDA before they can be sold.
Note:
For Class I products, the FDA only makes a public announcement after the company submits relevant documents to the FDA, and does not issue relevant certificates to the company. The whole process only takes 7 to 15 days.
For class Ⅱ and Ⅲ devices, the enterprise must submit PMN or PMA. FDA will give the enterprise formal market access approval letter at the same time of the announcement(Clearance),That allows companies to sell their products directly in the U.S. medical device market under their own name. The decision to conduct an on-site GMP inspection during the application process is determined by FDA based on a combination of factors, including product risk level, regulatory requirements and market feedback. The FDA registration process for Class II products can take anywhere from six months to a year.
We can clearly see that the reason FDA is not certification, but registration or registration, is because FDA does not issue any certificates to companies. The so-called certificate on the market will be failed. We can pass the FDA “certificate” like below, because our time is expensive.

Why are there so many different FDA versions?
You can't really blame the Photoshop people.Because the FDA has so many different testing agencies, all 50 states have them.And the FDA correspondence from each agency is different, their letters represent their respective institutions, that's why the FDA doesn't look the same version. You can't use that to tell if it's genuine or not.
Below is the FDA Clearance from Hubei Woozon Healthcare Co. Ltd. This is real FDA version.
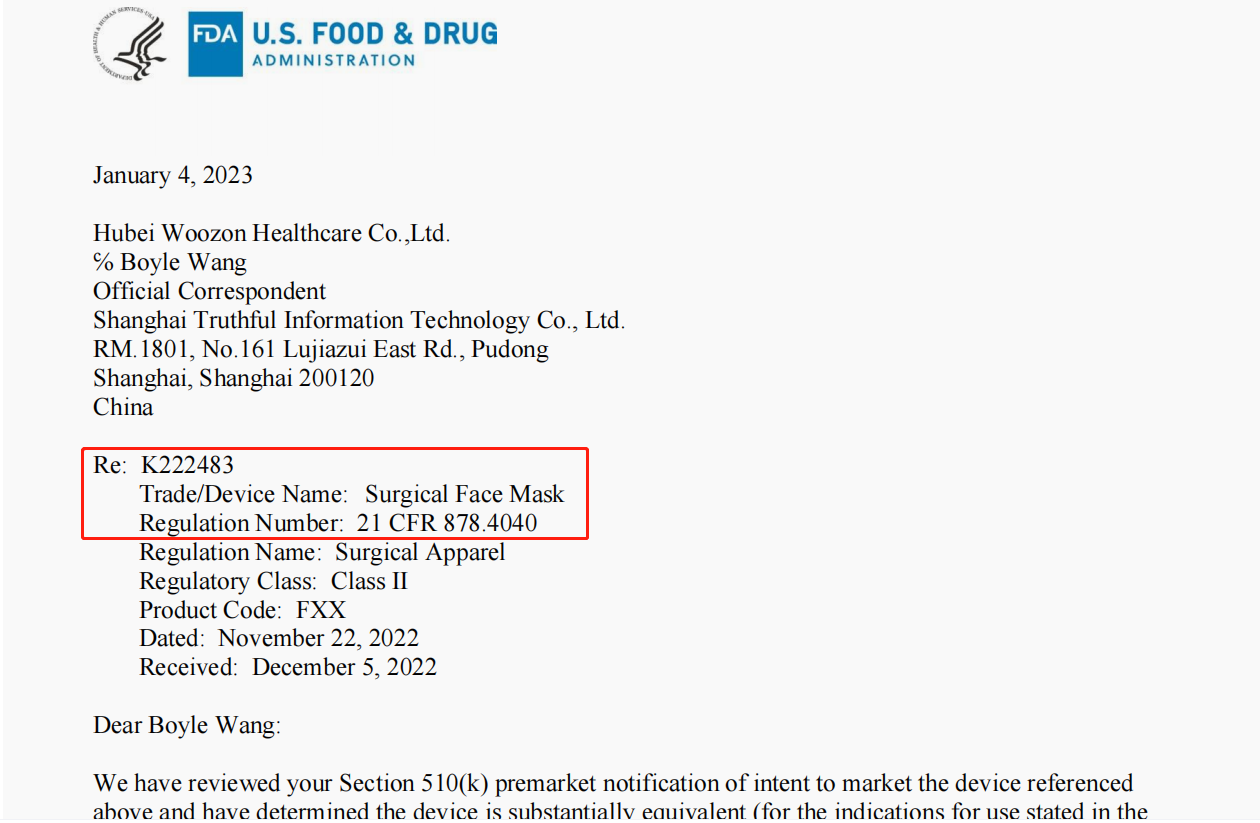
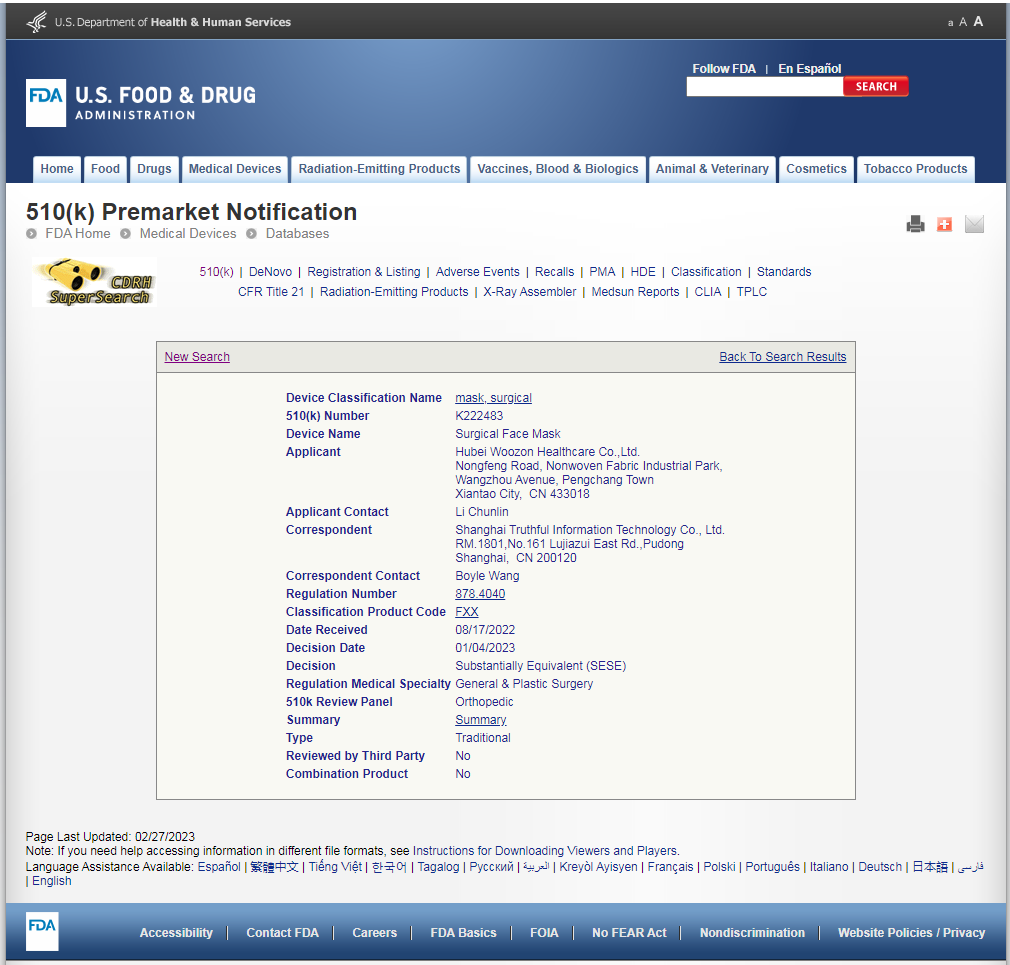
How to confirm the FDA is belong to Hubei Woozon Healthcare Co. Ltd and it is true?
Please see below steps.
1. Go to FDA website
https://www.fda.gov/
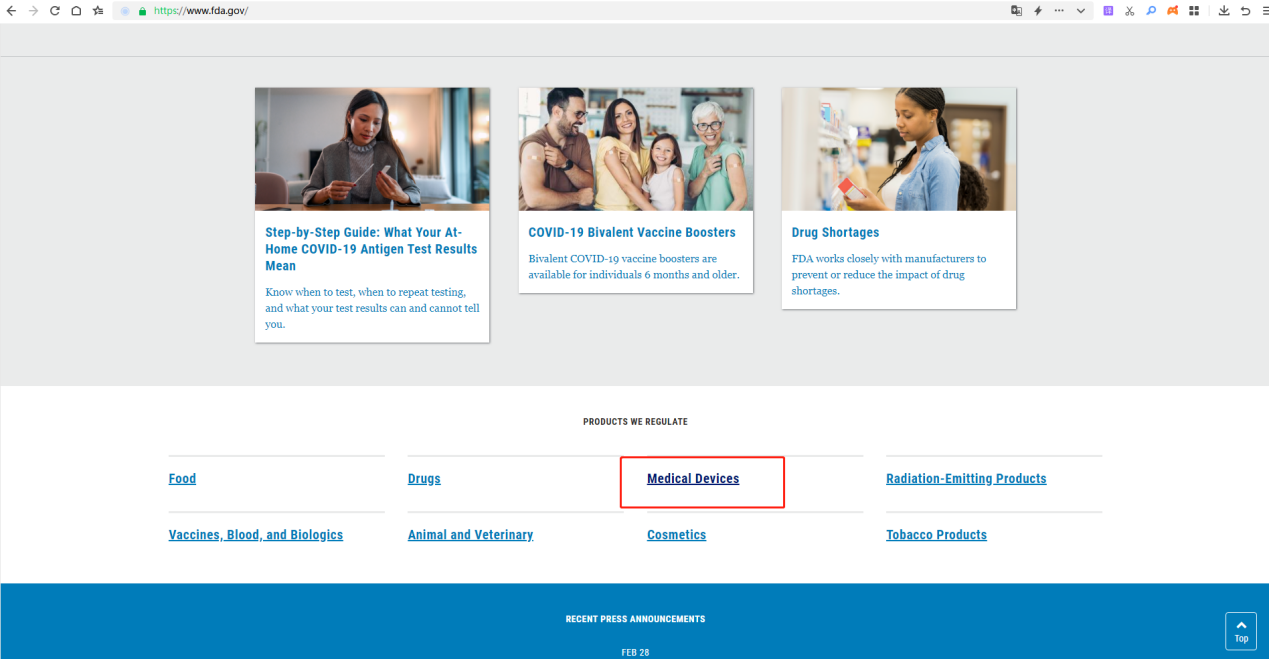
2.Go to the Medical Device page and click on it.
3.Find “510(k) Premarket Notification”and click it.
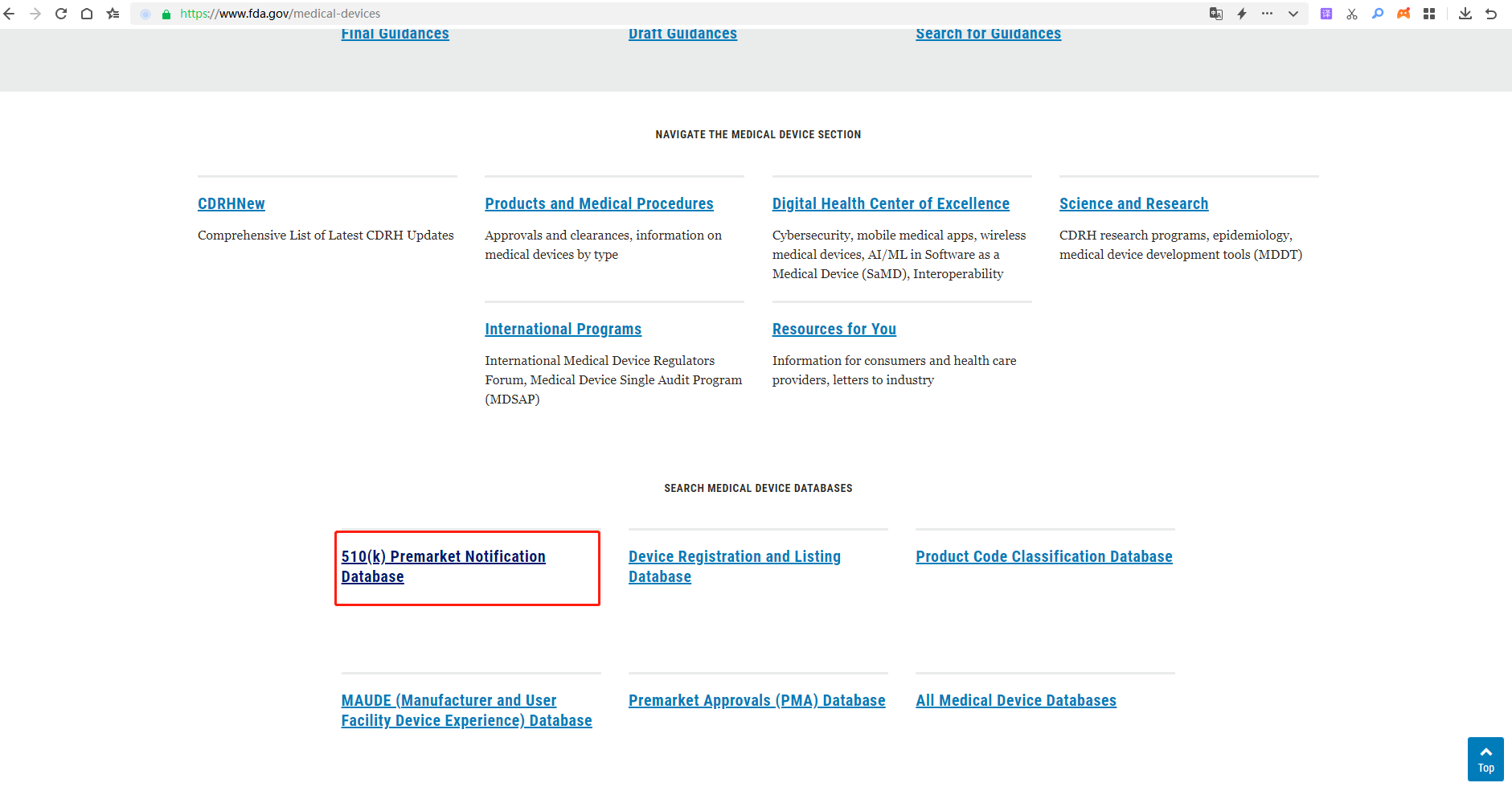
4.Enter the K number on the FDA Clearance. K222483
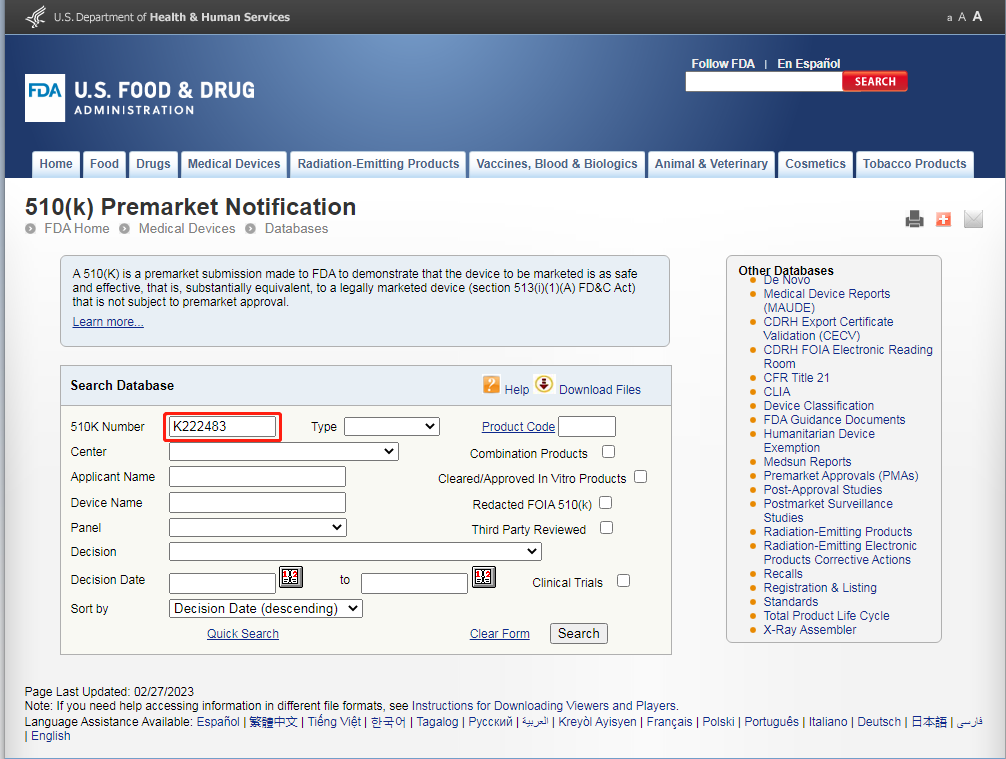
5.In the below, the registered applicant is the same as the registered product and registration certificate.
Scan the QR code to read on your phone
HOT INFO
WOOZON HEALTHCARE
Create more broad value and higher satisfaction for new and old customers

Tel:0086 19971586848
E-mail:info@hbwoozon.com
Add: Nongfeng Road, Nonwovens Industrial Park, Wangzhou Avenue, Pengchang Town, Xiantao City







